8.0 Solar Energy, Heat and Heat Transfer
Professor Jeremy Patrich MA and Laura J. Brown
Before we can understand the elements of weather and climate we need to know about their energy source and how that energy is transferred.
The Sun and Our Solar System
Our solar system is made up of the Sun, and the objects that orbit the sun. These objects are the planets and dwarf planets, their satellites, and many, many smaller elements, like asteroids or comets. All of these objects move, and we can see these movements. For example, we see the Sunrise in the eastern sky in the morning move overhead during the day and then set in the western sky in the evening. We observe star patterns change in the night sky at different times of the year. When ancient people made these observations, they imagined that the sky was moving while the Earth stood still. In 1543, Nicolaus Copernicus proposed a radically different idea: The Earth and the other planets make regular revolutions around the Sun. He also suggested that the Earth rotates once a day on its axis. Copernicus’ idea slowly gained acceptance and today we base our view of motions in the solar system on his work. We also now know that everything in the universe is moving.
Earth’s Axis, Rotation and Orbit
The Earth rotates once on its axis (Figure 8.0) about every 24 hours. If you were to look at Earth from above the North Pole, it would be spinning counterclockwise. As the Earth rotates, observers on Earth see the Sun moving across the sky from east to west with the beginning of each new day. We often say that the Sun is “rising” or “setting”, but it is the Earth’s rotation that gives us the perception of the Sun rising or setting over the horizon. When we look at the Moon or the stars at night, they also seem to rise in the east and set in the west. Earth’s rotation is also responsible for this. As Earth turns, the Moon and stars change position in our sky.
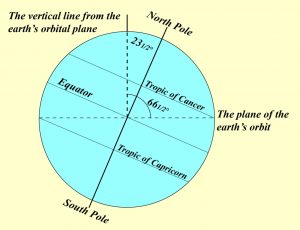
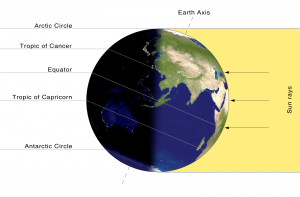
Another effect of Earth’s rotation is that we have a cycle of daylight and darkness every day. Because the Earth rotates, one side of Earth faces the Sun and experiences daylight, and the opposite side (facing away from the Sun) experiences darkness or nighttime. Since the Earth completes one rotation in about 24 hours, this is the time it takes to complete one day-night cycle. As the Earth rotates, different places on Earth experience sunset and sunrise at different times. As you move away from the equator towards the poles, summer and winter days have different amounts of daylight hours in a day. For example, in the Northern Hemisphere, we begin summer on or around June 21st which is the summer solstice. At this point, the Earth’s the North Pole is pointed directly toward the Sun (Figure 8.1). Therefore, areas north of the equator experience longer days and shorter nights because the northern half of the Earth is pointed toward the Sun. Since the southern half of the Earth is pointed away from the Sun at that point, they have the opposite effect, longer nights and shorter days.
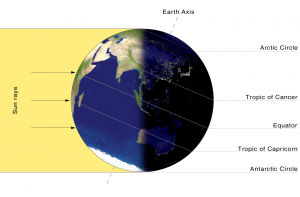
For people in the Northern Hemisphere, winter begins on or around December 21st, which is the winter solstice. At this point, it is Earth’s South Pole that is tilted toward the Sun (Figure 8.2), and so there are shorter days and longer nights for those who are north of the equator.
Energy from the Sun
Solar energy is radiant energy from the Sun. This radiant energy is emitted as part of the electromagnetic spectrum. The electromagnetic spectrum is classified regions based on the wavelength of the radiant energy. The shorter the wavelength the higher its energy. Going from shortest to longest the seven classifications are gamma rays, x-rays, ultraviolet radiation, visible light, infrared radiation, microwaves and radio waves. The shortest wavelengths, gamma, x-ray and some ultraviolet are filtered out by the upper parts of Earth’s atmosphere. About 44% of solar radiation reaching the Earth’s surface is in the visible light wavelengths, ultraviolet radiation accounts for 7% and the remaining energy is the longest wavelength, infrared. Most objects radiate infrared energy, which we feel as heat.
Incoming shortwave radiation
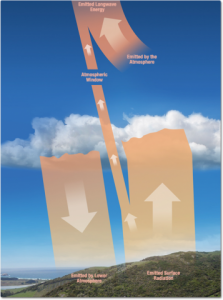
Incoming solar radiation (shortwave radiation) travels through space and enters our atmosphere. Some of this radiation is reflected off clouds, some is absorbed by atmospheric gases and some reaches the Earth’s surface (Figure 8.3). Shortwave radiation received at the Earth’s surface is either reflected or absorbed and converted into heat. The absorption of the incoming shortwave radiation by atmospheric particles or the Earth’s surface results in heat that is emitted as longwave radiation.

Source: NASA Science, share the science https://science.nasa.gov/ems/13_radiationbudget
Emitted longwave radiation
Heat resulting from the absorption of incoming shortwave radiation is emitted as longwave radiation. Most of this emitted longwave radiation warms the lower atmosphere, which in turn warms our planet’s surface. But the radiation from the warmed upper atmosphere and a small amount from the heated surface radiates out into space (Figure 8.4).
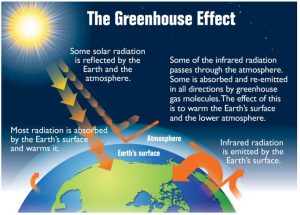
The Greenhouse Effect
Greenhouse gases in the atmosphere (such as water vapour, carbon dioxide, methane and other reactive gases) absorb most of the Earth’s emitted longwave infrared radiation, which heats the lower atmosphere. In turn, the warmed atmosphere emits longwave radiation, some of which radiates toward the Earth’s surface, keeping our planet warm and generally comfortable. Increasing concentrations of greenhouse gases such as carbon dioxide and methane increase the temperature of the lower atmosphere by restricting the outward passage of emitted radiation, resulting in “global warming,” or, more broadly, global climate change (Figure 8.5).
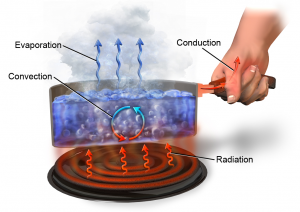
Heat Transfer
Heat always moves from hotter objects towards colder objects. The fundamental modes of heat transfer between objects are radiation, conduction, convection, advection and latent heat (Figure 8.6). Radiation is the transfer of energy via electromagnetic waves and is unique because these waves can travel through space without an intervening medium between the emitter and absorber. For example, the heat you feel from a fire. Conduction is the transfer of heat within a substance or when objects are touching each other. This is a molecule-to-molecule interaction. In Figure 8.6 when the pot is put onto the stove’s heating element, the molecules at the bottom of the pot absorb energy and begin to vibrate faster. As they do their energy increases and they begin to bump into adjacent molecules and in doing so transfer some of their energy. In this way, the heat is transferred from the bottom of the pot up the sides and along its handle. Convection is the transfer of heat by circulation in a fluid and heat is transferred by the movement of the fluid in the vertical (up/down) direction. This type of heat transfer takes place in both liquids and gases. Latent Heat is the heat energy required to change a substance from one state to another. In this example, liquid water is converted into water vapour by evaporation and latent heat is stored in the vapour. Advection is another form of heat transfer but is not included in this figure. It is the transfer of heat from one place to another by the wind.