Dairy Products
30 Butter Manufacture
Butter is essentially the fat of the milk. It is usually made from sweet cream and is salted. However, it can also be made from acidulated or bacteriologically soured cream and saltless (sweet) butters are also available. Well into the 19th century butter was still made from cream that had been allowed to stand and sour naturally. The cream was then skimmed from the top of the milk and poured into a wooden tub. Buttermaking was done by hand in butter churns. The natural souring process is, however, a very sensitive one and infection by foreign micro-organisms often spoiled the result. Today’s commercial buttermaking is a product of the knowledge and experience gained over the years in such matters as hygiene, bacterial acidifying and heat treatment, as well as the rapid technical development that has led to the advanced machinery now used. The commercial cream separator was introduced at the end of the 19th century, the continuous churn had been commercialized by the middle of the 20th century.
Definitions and Standards
Milkfat
the lipid components of milk, as produced by the cow, and found in commercial milk and milk-derived products, mostly comprised of triglyceride.
Butterfat
Almost synonymous with milkfat; all of the fat components in milk that are separable by churning.
Anhydrous Milkfat (AMF)
The commercially- prepared extraction of cow’s milkfat, found in bulk or concentrated form (comprised of 100% fat, but not necessarily all of the lipid components of milk).
Butteroil
Synonymous with anhydrous milkfat; (conventional terminology in the fats and oils field differentiates an oil from a fat based on whether it is liquid at room temp. or solid, but very arbitrary).
Butter
A water-in-oil emulsion, comprised of >80% milkfat, but also containing water in the form of tiny droplets, perhaps some milk solids-not-fat, with or without salt (sweet butter); texture is a result of working/kneading during processing at appropriate temperatures, to establish fat crystalline network that results in desired smoothness (compare butter with melted and recrystallized butter); used as a spread, a cooking fat, or a baking ingredient.
The principal constituents of a normal salted butter are fat (80 – 82%), water (15.6 – 17.6%), salt (about 1.2%) as well as protein, calcium and phosphorous (about 1.2%). Butter also contains fat-soluble vitamins A, D and E.
Butter should have a uniform colour, be dense and taste clean. The water content should be dispersed in fine droplets so that the butter looks dry. The consistency should be smooth so that the butter is easy to spread and melts readily on the tongue.
Overview of the Buttermaking Process
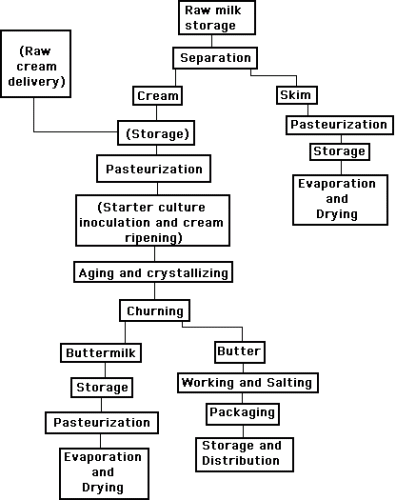
The buttermaking process involves quite a number of stages. The continuous buttermaker has become the most common type of equipment used.
The cream can be either supplied by a fluid milk dairy or separated from whole milk by the butter manufacturer. The cream should be sweet (pH >6.6, TA = 0.10 – 0.12%), not rancid and not oxidized.
If the cream is separated by the butter manufacturer, the whole milk is preheated to the required temperature in a milk pasteurizer before being passed through a separator. The cream is cooled and led to a storage tank where the fat content is analyzed and adjusted to the desired value, if necessary. The skim milk from the separator is pasteurized and cooled before being pumped to storage. It is usually destined for concentration and drying.
From the intermediate storage tanks, the cream goes to pasteurization at a temperature of 95oC or more. The high temperature is needed to destroy enzymes and micro-organisms that would impair the keeping quality of the butter.
If ripening is desired for the production of cultured butter, mixed cultures of S. cremoris, S. lactis diacetyl lactis, Leuconostocs, are used and the cream is ripened to pH 5.5 at 21oC and then pH 4.6 at 13oC. Most flavour development occurs between pH 5.5 – 4.6. The colder the temperature during ripening the more the flavour development relative to acid production. Ripened butter is usually not washed or salted.
In the aging tank, the cream is subjected to a program of controlled cooling designed to give the fat the required crystalline structure. The program is chosen to accord with factors such as the composition of the butterfat, expressed, for example, in terms of the iodine value which is a measure of the unsaturated fat content. The treatment can even be modified to obtain butter with good consistency despite a low iodine value, i.e. when the unsaturated proportion of the fat is low.
As a rule, aging takes 12 – 15 hours. From the aging tank, the cream is pumped to the churn or continuous buttermaker via a plate heat exchanger which brings it to the requisite temperature. In the churning process the cream is violently agitated to break down the fat globules, causing the fat to coagulate into butter grains, while the fat content of the remaining liquid, the buttermilk, decreases.
Thus the cream is split into two fractions: butter grains and buttermilk. In traditional churning, the machine stops when the grains have reached a certain size, whereupon the buttermilk is drained off. With the continuous buttermaker the draining of the buttermilk is also continuous.
After draining, the butter is worked to a continuous fat phase containing a finely dispersed water phase. It used to be common practice to wash the butter after churning to remove any residual buttermilk and milk solids but this is rarely done today.
Salt is used to improve the flavour and the shelf-life, as it acts as a preservative. If the butter is to be salted, salt (1-3%) is spread over its surface, in the case of batch production. In the continuous buttermaker, a salt slurry is added to the butter. The salt is all dissolved in the aqueous phase, so the effective salt concentration is approximately 10% in the water.
After salting, the butter must be worked vigorously to ensure even distribution of the salt. The working of the butter also influences the characteristics by which the product is judged – aroma, taste, keeping quality, appearance and colour. Working is required to obtain a homogenous blend of butter granules, water and salt. During working, fat moves from globular to free fat. Water droplets decrease in size during working and should not be visible in properly worked butter. Overworked butter will be too brittle or greasy depending on whether the fat is hard or soft. Some water may be added to standardize the moisture content. Precise control of composition is essential for maximum yield.
The finished butter is discharged into the packaging unit, and from there to cold storage.
The background science of butter churning
Milk fat is comprised mostly of triglycerides, with small amounts of mono- and diglycerides, phospholipids, glycolipids, and lipo-proteins. The trigylcerides (98% of milkfat) are of diverse composition with respect to their component fatty acids, approximately 40% of which are unsaturated fat firmness varies with chain length, degree of unsaturation, and position of the fatty acids on the glycerol. Fat globules vary from 0.1 – 10 micron in diameter. The fat globule membrane is comprised of surface active materials: phospholipids and lipoproteins.
Fat globules typically aggregate in three ways:
- flocculation
- coalescence
- partial coalescence
Whipping and Churning
Many milk products foam easily. Skim milk foams copiously with the amount of foam being very dependent on the amount of residual fat – fat depresses foaming. The foaming agents are proteins, the amount of proteins in the foam are proportional to their contents in milk. Foaming is decreased in heat treated milk, possibly because denaturated whey proteins produce a more brittle protein layer at the interface. Fats tend to spread over the air-water interface and destabilize the foam; very small amounts of fats (including phospholipids) can destabilize a foam.
During the interaction of fat globules with air bubbles the globule may also be disrupted (this is the only way that fat globules can be disrupted without considerable energy input). Disruption of the fat globule by interaction between the fat globule and air bubbles is rare except in the case of newly formed air bubbles where the air-water interfacial layer is still thin. If part of the fat globule is solid, churning will result, hence the term “flotation churning” -from repeated rupturing of air bubbles and resulting coalescence of the adsorbed fat.
In spite of the above comments on the destabilization of foams by fat, milk fat is essential for the formation of stable whipped products which depend on the interaction between fat globules, air bubbles and plasma components (esp. proteins).
When cream is beaten air cells form more slowly partly because of higher viscosity and partly because the presence of fat causes immediate collapse of most of the larger bubbles. If most of the fat is liquid (high temperature) the fat globule membrane is not readily punctured and churning does not occur -at cold temperature where solid fat is present, churning (clumping) of the fat globule takes place. Clumps of globules begin to associate with air bubbles so that a network of air bubbles and fat clumps and globules form entrapping all the liquid and producing a stable foam. If beating continues the fat clumps increase in size until they become too large and too few to enclose the air cells, hence air bubbles coalesce, the foam begins to “leak” and ultimately butter and butter milk remain.
Crystallizing of the milkfat during aging
The relative amounts of fatty acids with high melting point determine whether the fat will be hard or soft. Soft fat has a high content of low-melting fatty acids and at room temperature this fat has a large continuous fat phase with a low solid phase, i.e. crystallized, high-melting fat. On the other hand, in a hard fat, the solid phase of high-melting fat is much larger than the continuous fat phase of low-melting fatty acids.
In buttermaking, if the cream is always subjected to the same heat treatment it will be the chemical composition of the milk fat that determines the butter’s consistency. A soft milk fat will make a soft and greasy butter, whereas butter from hard milk fat will be hard and stiff. If, however, the heat treatment is modified to suit the iodine value of the fat, the consistency of the butter can be optimized. For the heat treatment regulates the size of the fat crystals, and the relative amounts of solid fat and the continuous phase – the factors that determine the consistency of the butter.
Pasteurization causes the fat in the fat globules to liquefy. And when the cream is subsequently cooled a proportion of the fat will crystallize. If cooling is rapid, the crystals will be many and small; if gradual the yield will be fewer but larger crystals. The more violent the cooling process, the more will be the fat that will crystallize to form the solid phase, and the less the liquid fat that can be squeezed out of the fat globules during churning and working.
The crystals bind the liquid fat to their surface by adsorption. Since the total surface area is much greater if the crystals are many and small, more liquid fat will be adsorbed than if the crystals were larger and fewer. In the former case, churning and working will press only a small proportion of the liquid fat from the fat globules. The continuous fat phase will consequently be small and the butter firm. In the latter case, the opposite applies. A larger amount of liquid fat will be pressed out; the continuous phase will be large and the butter soft.
So by modifying the cooling program for the cream, it is possible to regulate the size of the crystals in the fat globules and in this way influence both the magnitude and the nature of the important continuous fat phase.
Tempering Treatment of Hard Fat. For optimum consistency where the iodine value is low, i.e. the butterfat is hard, as much as possible of the hardest fat must be converted to as few crystals as possible, so that little of the liquid fat is bound to the crystals. The liquid fat phase in the fat globules will thereby be maximized and much of it can be pressed out during churning and working, resulting in butter with a relatively large continuous phase of liquid fat and with the hard fat concentrated to the solid phase.
The program of treatment necessary to achieve this result comprises the following stages:
- rapid cooling to about 8°C and storage for about 2 hours at this temperature;
- heating gently to 20- 21°C and storage at this temperature for at least 2 hours (water at 27- 29°C is used for heating);
- cooling to about 16°C.
Cooling to about 8°C causes the formation of a large number of small crystals that bind fat from the liquid continuous phase to their surface.
When the cream is gently heated to 20- 21°C the bulk of the crystals melt, leaving only the hard fat crystals which, during the storage period at 20- 21°C, grow larger.
After 1 – 2 hours most of the hard fat has crystallized, binding little of the liquid fat. By dropping the temperature now to about 16°C, the hardest portion of the fat will be fixed in crystal form while the rest is liquefied. During the holding period at 16°C, fat with a melting point of 16°C or higher will be added to the crystals. The treatment has thus caused the high-melting fat to collect in large crystals with little adsorption of the low-melting liquid fat, so that a large proportion of the butter oil can be pressed out during churning and working.
Tempering Treatment of Medium Hard Fat. With an increase in the iodine value, the heating temperature is accordingly reduced from 20-21°C. Consequently a larger number of fat crystals will form and more liquid fat will be adsorbed than is the case with the hard fat program. For iodine values up to 39, the heating temperature can be as low as 15°C.
Tempering Treatment of Very Soft Fat. Where the iodine value is greater than 39-40 the “summer method” of treatment is used. After pasteurization the cream is cooled to 20°C. If the iodine value is around 39 – 40 the cream is cooled to about 8°C, and if 41 or greater to 6°C. It is generally held that aging temperatures below the 20°C level will give a soft butter.
Butter structure
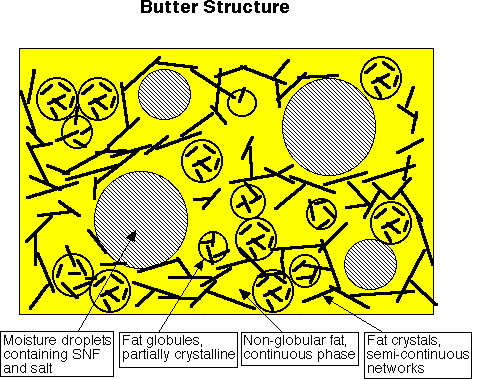
It should now be obvious from the discussions regarding the background science of churning and the crystallization processes that the structure of butter is quite complicated. The size and extent of crystal networks both within the globules and within the non-globular phases is controlled to a large extent by milkfat’s variable composition and by the aging process. The extent of globular versus non-globular fat is controlled to a large extent also by the amount of physical working applied to the butter post-churning.
Continuous Buttermaking
- traditional batch churning from 25- 35% mf. cream;
- continuous flotation churning from 30-50% mf. cream;
- the concentration process whereby “plastic” cream at 82% mf. is separated from 35% mf. cream at 55°C and then this oil-in-water emulsion cream is inverted to a water-in-oil emulsion butter with no further draining of buttermilk;
- the anhydrous milkfat process whereby water, SNF, and salt are emulsified into butter oil in a process very similar to margarine manufacture.
An optimum churning temperature must be determined for each type of process but is mainly dependent on the mean melting point and melting range of the lipids, as discussed above, i.e., 7-10°C in summer and 10-13°C in winter. If churning temperature is too warm or if the thermal cream aging cycle permits too much liquid fat, then a soft greasy texture results; if too cold or too much solid fat, then butter becomes too brittle.
Continuous Flotation Churns
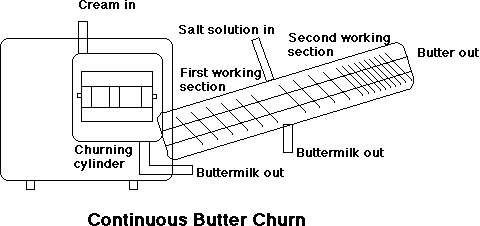
The cream is first fed into a churning cylinder fitted with beaters that are driven by a variable speed motor.
Rapid conversion takes place in the cylinder and, when finished, the butter grains and buttermilk pass on to a draining section. The first washing of the butter grains sometimes takes place en route – either with water or recirculated chilled buttermilk. The working of the butter commences in the draining section by means of a screw, which also conveys it to the next stage.
On leaving the working section the butter passes through a conical channel to remove any remaining buttermilk. Immediately afterwards, the butter may be given its second washing, this time by two rows of adjustable high-pressure nozzles. The water pressure is so high that the ribbon of butter is broken down into grains and consequently any residual milk solids are effectively removed. Following this stage, salt may be added through a high-pressure injector.
The third section in the working cylinder is connected to a vacuum pump. Here it is possible to reduce the air content of the butter to the same level as conventionally churned butter.
In the final or mixing section the butter passes a series of perforated disks and star wheels. There is also an injector for final adjustment of the water content. Once regulated, the water content of the butter deviates less than +/- 0.1%, provided the characteristics of the cream remain the same.
The finished butter is discharged in a continuous ribbon from the end nozzle of the machine and then into the packaging unit.
Concentration Method
- 30% fat cream pasteurized at 90°C
- degassed in a vacuum
- cooled to 45-70°C
- separated to 82% fat (“plastic” cream)
- the concentrate, still an O/W emulsion, is cooled to 8-13°C
- fat crystals forming in the tightly packed globules perforate the membranes, cause liquid fat leakage and rapid phase inversion
- contrast to mayonnaise, also a o/w emulsion at 82% fat but is winterized to prevent crystallization
- butter from this method contains all membrane material, therefore, more phospholipids
- no butter milk produced
- after phase inversion the butter is worked and salted.
Phase Separation
- prepare “plastic” cream (>80% fat)
- heat with agitation to destabilize emulsion
- separate oil from aqueous phase: 82 to 98% butter fat
- this butter oil is then blended with water, salt and milk solids in an emulsion pump and transferred to a scraped surface heat exchanger for cooling and to initiate crystallization
- further worked to develop crystal structure and texture
- process similar to margarine manufacture
- margarine has advantage of fat composition control to modify physical properties
- butter produced by phase separation contains few phospholipids.
Butter Yield Calculations
Separation efficiency (Es)
Es=![]()
where fs = skim fat as percent w/w
fm = milk fat as percent w/w
Separation efficiency depends on initial milk fat content and residual fat in the skim. Assuming optimum operation of the separator, the principal determining factor of fat loss to the skim is fat globule size. Modern separators should achieve a skim fat content of 0.04 – 0.07%.
Churning Efficiency (Ec)
Ec = 1 – fbm/fc
where fbm = buttermilk fat as percent w/w
fc = cream fat as percent w/w
Maximum acceptable fat loss in buttermilk is about 0.7% of churned fat corresponding to a churning efficiency of 99.3% of cream fat recovered in the butter. Churning efficiency is highest in the winter months and lowest in the summer months. Fat losses are higher in ripened butter due to a restructuring of the FGM (possibly involving crystallization of high melting triglycerides on the surface of the globules). If churning temperature is too high, churning occurs more quickly but fat loss in buttermilk increases. For continuous churns assuming 45% cream, churning efficiency should be 99.61 – 99.42%.
Composition Overrun
= (Kg butter made – Kg fat churned)/Kg fat churned x 100 %
% Composition Overrun
= (100 – % fat in butter)/% fat x 100 %
Package Fill Control
An acceptable range for 25 kg butter blocks is 0.2 – 0.4% overfill. Overfill on 454 g prints is about 0.6%.
Other factors affecting yield
- shrinkage due to leaky butter (improperly worked).
- shrinkage due to moisture loss; avoided by aluminum wrap.
- loss of butter remnants on processing equipment; % loss minimal in large scale continuous processing.
Plant Overrun
Separation Efficiency 98.85
Churning Efficiency 99.60
Composition overrun (% fat) 23.30
Package overfill 0.20
These values can be used to predict the expected yield of butter per kg of milk or kg of milk fat received.
Example
0.05% m.f. in skim
40% m.f. in cream
0.3% m.f. in buttermilk
81.5% m.f. in butter
Es = 1 – .05/3.6 = 98.6
Ec = 1 – .3/40 = 99.25
% Composition Overrun = (100-81.5)/81.5 = 22.7%
If 100 kg of milk was used, 8.9 kg of cream would be produced (from a Pearson Square mass balance) and 4.35 kg butter would be produced from that. This is the theoretical yield based on no losses. The mass balance of fat shows that 98.3% of the fat ended up in the butter, 0.4% of the fat ended up in the buttermilk and 1.3% of the fat ended up in the skim.
The % Churn Overrun = (4.35 – 3.6)/3.6 = 20.8%
Whipped Butter
Anhydrous Milkfat (“butter oil”)
The processes for the production of anhydrous fat, using cream as the raw material, are based on the emulsion splitting principle. In brief, the processes consist of the cream first being concentrated to 75% fat or greater, in two stages. In both of these stages, the fat is concentrated in a hermetic solids-ejecting separator. The fat globules are then broken down mechanically, so that phase inversion occurs and the fat is liberated. This forms a continuous fat phase containing dispersed water droplets, which can be separated from the fat phase by centrifugation. This is similar to the concentration method for buttermaking, with the addition of the mechanical rupture of the emulsion and additional separator for removal of the residual water phase.
One of the key machines in the system is the mechanical device for phase inversion. This can be in the form of a centrifugal separator equipped with a serrated disc. The disc breaks down the emulsion, so that the liquid leaving the machine is a continuous oil phase, with dispersed water droplets and buttermilk. Larger equipment could be equipped with a motor-driven serrated disc or with a homogenizer. After phase inversion, the fat is concentrated to 99.5% or greater in a hermetic separator.
Fractionation of anhydrous milk fat
Milk fat is a complicated mixture of triglycerides that contain numerous fatty acids of varying carbon chain lengths and degrees of saturation. The proportions of the various fatty acids present will also vary depending on the conditions surrounding the production of milk.
One method of milkfat fraction is by thermal treatment. The mixture can be separated into fractions on the basis of their melting point. The technique consists of melting the entire quantity of fat and then cooling it down to a predetermined temperature. The triglycerides with the higher melting point will then crystallize and settle out.
In the modern thermal fractionation method, sedimentation by gravity is replaced by centrifugal separation. Since a modern separator generates a force that is thousands of times greater than the force of gravity and since the sedimentation distances are very short, the process is incomparably faster. The crystallizing stage can also be accelerated, since the crystals need not be large if centrifugal separation is employed.
Fractionation of milkfat can also be accomplished by supercritical fluid extraction techniques.
Some of this material has been condensed from the Tetrapak Dairy Processing Handbook, with permission.

