Dairy Processing
24 Membrane Processing
Membrane processing is a technique that permits concentration and separation without the use of heat. Particles are separated on the basis of their molecular size and shape with the use of pressure and specially designed semi-permeable membranes. There are some fairly new developments in terms of commercial reality and is gaining readily in its applications:
- proteins can be separated in whey for the production of whey protein concentrate (WPC)
- milk can be concentrated prior to cheesemaking at the farm level
- apple juice and wine can be clarified
- waste treatment and product recovery is possible in edible oil, fat, potato, and fish processing
- fermentation broths can be clarified and separated
- whole egg and egg white ultrafiltration as a pre-concentration prior to spray drying
The following topics will be covered in this section:
Principle of Operation
Reverse Osmosis, Ultra-and Diafiltration and Microfiltration
Hardware Design
Electrodialysis
Ion Exchange
Principle of Operation
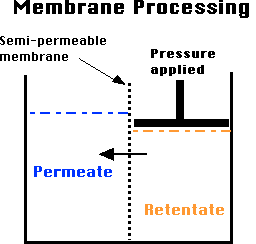
Reverse Osmosis, Ultra- and Diafiltration and Microfiltration
Ultrafiltration (UF) is a membrane separation process, driven by a pressure gradient, in which the membrane fractionates dissolved and dispersed components of a liquid as a function of their solvated size and structure. The membrane configuration is usually cross-flow. In UF, the membrane pore size is larger than RO, thus allowing some components to pass through the pores with the water. It is a separation/ fractionation process using a 10,000 MW cutoff, 40 psig, and temperatures of 50-60°C with polysulfone membranes. In UF of skim milk, lactose and minerals are not fractionated; for example, in the retentate would be 100% of the protein but the same % of lactose and free minerals in solution (in the water phase) as existed in the skim.
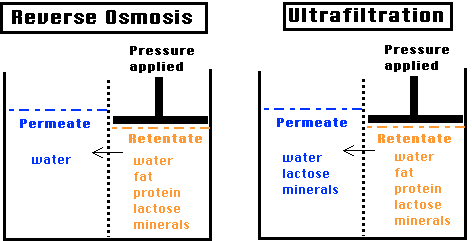
This can be visualized with another schematic, as follows, which may be more informative:
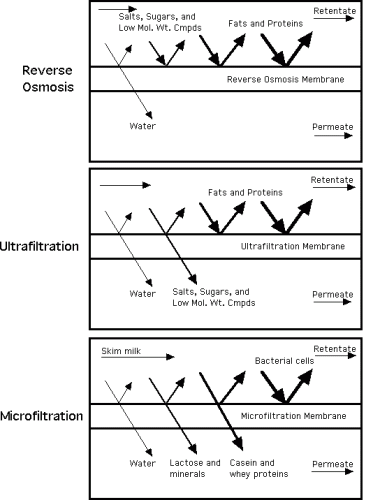
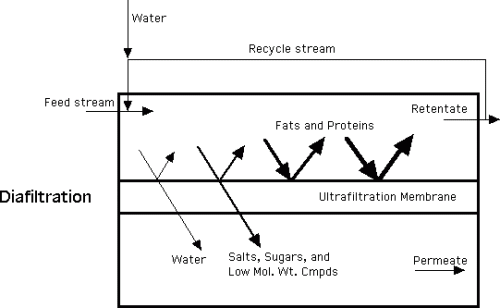
Diafiltration is a specialized type of ultrafiltration process in which the retentate is diluted with water and re-ultrafiltered, to reduce the concentration of soluble permeate components and increase further the concentration of retained components. This schematic shows the process of diaflitration, as a step in ultrafiltration.
Microfiltration (MF) (see diagram above) is a membrane separation process similar to UF but with even larger membrane pore size allowing particles in the range of 0.2 to 2 micrometers to pass through. The pressure used is generally lower than that of UF process. The membrane configuration is usually cross-flow. MF is used in the dairy industry for making low-heat sterile milk as proteins may pass through but bacteria do not. The permeate of skim milk is used as “bacteria-free” skim (although thee is no fail-safe guarantee as there could be pin-holes in the membrane) since all of the milk components will pass through the membrane. In that case, the retentate, skim enriched in bacteria, is high-heat treated. MF skim can then be standardized for fat with high heat-treated cream.
Hardware Design
Tubes of membrane with a diameter of 1/2 to 1 inch and length to 12 ft. are encased in reinforced fibreglass or enclosed inside a rigid PVC or stainless steel shell. As the feed solution flows through the membrane core, the permeate passes through the membrane and is collected in the tubular housing. Imagine 12 ft long straws!
Hollow Fibre:
Similar to open tubular, but the cartridges contain several hundred very small (1 mm diam) hollow membrane tubes or fibres. As the feed solution flows through the open cores of the fibres, the permeate is collected in the cartridge area surrounding the fibres.
Plate and Frame:
This system is set up like a plate heat exchanger with the retentate on one side and the permeate on the other. The permeate is collected through a central collection tube.
Spiral Wound:
This design tries to maximize surface area in a minimum amount of space. It consists of consecutive layers of large membrane and support material in an envelope type design rolled up around a perforated steel tube.
Electrodialysis
Principles of operation:
Under the influence of an electric field, ions move in an aqueous solution. The ionic mobility is directly proportioned to specific conductivity and inversely proportioned to number of molecules in solution. ~3-6 x 102 mm/sec.
Charged ions can be removed from a solution by synthetic polymer membranes containing ion exchange groups. Anion exchange membranes carry cationic groups which repel cations and are permeable to anions, and cation exchange membranes contain anionic groups and are permeable only to cations.
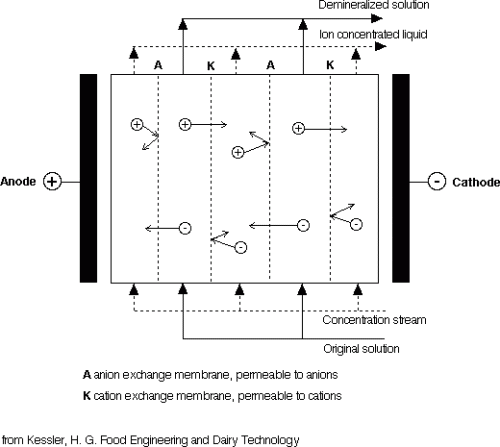
Electrodialysis membranes are comprised of polymer chains – styrene-divinyl benzene made anionic with quaternary ammonium groups and made cationic with sulphonic groups. 1-2V is then applied across each pair of membranes.
Electrodialysis process:
Anion and cation exchange membranes are arranged alternately in parallel between an anode and a cathode (see this schematic diagram to the right). The distance between the membranes is 1mm or less. A plate and frame arrangement similar to a plate heat exchanger or a plate filter is used. The solution to be demineralized flows through gaps between the two types of membranes. Each type of membrane is permeable to only one type of ion. Thus, the anions leave the gap in the direction of the anode and cations leave in the direction of the cathode. Both are then taken up by a concentrating stream.
Problems:
Concentration polarization. Deposits on membrane surfaces, e.g. proteins – pH control is important. Prior concentration of whey, to 20% TS, is necessary before electrodialysis.
Ion Exchange
Fractionation may also be accomplished using ion exchange processing. It relies on inert resins (cellulose or silica based) that can adsorb charged particles at either end of the pH scale. The design can be a batch type, stirred tank or continuous column. The column is more suitable for selective fractionation. Whey protein isolate (WPI), with a 95% protein content, can be produced by this method. Following adsorption and draining of the de-proteined whey, the pH or charge properties are altered and proteins are eluted. Protein is recovered from the dilute stream through UF and drying. Selective resins may be used for fractionated protein products or enriched in fraction allow tailoring of ingredients.

