Dairy Chemistry and Physics
8 Milk Lipids- Chemical Properties; Physical Properties; Structure and Fat Globules; Functional Properties
Chemical Properties
The fat content of milk is of economic importance because milk is sold on the basis of fat. Milk fatty acids originate either from microbial activity in the rumen, and transported to the secretory cells via the blood and lymph, or from synthesis in the secretory cells. The main milk lipids are a class called triglycerides which are comprised of a glycerol backbone binding up to three different fatty acids. The fatty acids are composed of a hydrocarbon chain and a carboxyl group. The major fatty acids found in milk are:
Long chain
- C14 – myristic (11%)
- C16 – palmitic (26%)
- C18 – stearic (10%)
- C18:1 – oleic (20%)
Short chain
- C4 – butyric* (11%)
- C6 – caproic
- C8 – caprylic
- C10 – capric
* butyric fatty acid is specific for milk fat of ruminant animals and is responsible for the rancid flavour when it is cleaved from glycerol by lipase action.
Saturated fatty acids (no double bonds), such as myristic, palmitic, and stearic make up two thirds of milk fatty acids. Oleic acid is the most abundant unsaturated fatty acid in milk with one double bond. While the cis form of geometric isomer is the most common found in nature, approximately 5% of all unsaturated bonds are in the trans position as a result of rumen hydrogenation.
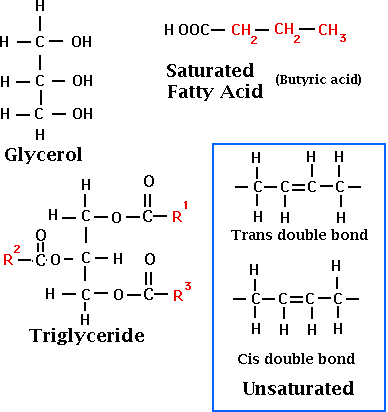
Triglycerides account for 98.3% of milkfat. The distribution of fatty acids on the triglyceride chain, while there are hundreds of different combinations, is not random. The fatty acid pattern is important when determining the physical properties of the lipids. In general, the SN1 position binds mostly longer carbon length fatty acids, and the SN3 position binds mostly shorter carbon length and unsaturated fatty acids. For example:
- C4 – 97% in SN3
- C6 – 84% in SN3
- C18 – 58% in SN1
The small amounts of mono- , diglycerides, and free fatty acids in fresh milk may be a product of early lipolysis or simply incomplete synthesis. Other classes of lipids include phospholipids (0.8%) which are mainly associated with the fat globule membrane, and cholesterol (0.3%) which is mostly located in the fat globule core.
Physical Properties
The physical properties of milkfat can be summarized as follows:
- density at 20° C is 915 kg m-3
- refractive index (589 nm) is 1.462 which decreases with increasing temperature
- solubility of water in fat is 0.14% (w/w) at 20°C and increases with increasing temperature
- thermal conductivity is about 0.17 J m-1 s-1 K-1 at 20°C
- specific heat at 40° C is about 2.1 kJ kg-1 K-1
- electrical conductivity is <10-12 ohm-1 cm-1
- dielectric constant is about 3.1
At room temperature, the lipids are solid, therefore, are correctly referred to as “fat” as opposed to “oil” which is liquid at room temperature. The melting points of individual triglycerides ranges from -75° C for tributyric glycerol to 72° C for tristearin. However, the final melting point of milkfat is at 37° C because higher melting triglycerides dissolve in the liquid fat. This temperature is significant because 37° C is the body temperature of the cow and the milk would need to be liquid at this temperature. The melting curves of milkfat are complicated by the diverse lipid composition:
trans unsaturation increases melting points
odd-numbered and branched chains decrease melting points
Crystallization of milkfat largely determines the physical stability of the fat globule and the consistency of high-fat dairy products, but crystal behaviour is also complicated by the wide range of different triglycerides. There are four forms that milkfat crystals can occur in; alpha, ß , ß ‘ 1, and ß ‘ 2, however, the alpha form is the least stable and is rarely observed in slowly cooled fat.
Fat Globules
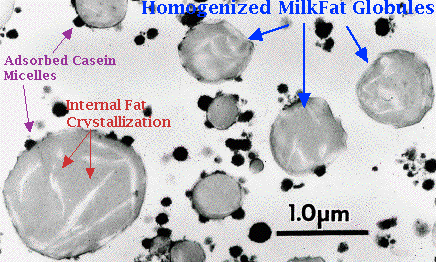
More than 95% of the total milk lipid is in the form of a globule ranging in size from 0.1 to 15 um in diameter. These liquid fat droplets are covered by a thin membrane, 8 to 10 nm in thickness, whose properties are completely different from both milkfat and plasma. The native fat globule membrane (FGM) is comprised of apical plasma membrane of the secretory cell which continually envelopes the lipid droplets as they pass into the lumen. The major components of the native FGM, therefore, is protein and phospholipids. The phospholipids are involved in the oxidation of milk. There may be some rearrangement of the membrane after release into the lumen as amphiphilic substances from the plasma adsorb onto the fat globule and parts of the membrane dissolve into either the globule core or the serum. The FGM decreases the lipid-serum interface to very low values, 1 to 2.5 mN/m, preventing the globules from immediate flocculation and coalescence, as well as protecting them from enzymatic action.
It is well known that if raw milk or cream is left to stand, it will separate. Stokes’ Law predicts that fat globules will cream due to the differences in densities between the fat and plasma phases of milk. However, in cold raw milk, creaming takes place faster than is predicted from this fact alone. IgM, an immunoglobulin in milk, forms a complex with lipoproteins. This complex, known as cryoglobulinprecipitates onto the fat globules and causes flocculation. This is known as cold agglutination. As fat globules cluster, the speed of rising increases and sweeps up the smaller globules with them. The cream layer forms very rapidly, within 20 to 30 min., in cold milk.
Homogenization of milk prevents this creaming by decreasing the diameter and size distribution of the fat globules, causing the speed of rise to be similar for the majority of globules. As well, homogenization causes the formation of a recombined membrane which is much similar in density to the continuous phase.
Recombined membranes are very different than native FGM. Processing steps such as homogenization, decreases the average diameter of fat globule and significantly increases the surface area. Some of the native FGM will remain adsorbed but there is no longer enough of it to cover all of the newly created surface area. Immediately after disruption of the fat globule, the surface tension raises to a high level of 15 mN/m and amphiphilic molecules in the plasma quickly adsorb to the lipid droplet to lower this value. The adsorbed layers consist mainly of serum proteins and casein micelles.
Fat Destabilization
While homogenization is the principal method for achieving stabilization of the fat emulsion in milk, fat destabilization is necessary for structure formation in butter, whipping cream and ice cream. Fat destabilization refers to the process of clustering and clumping (partial coalescence) of the fat globules which leads to the development of a continuous internal fat network or matrix structure in the product. Fat destabilization (sometimes “fat agglomeration”) is a general term that describes the summation of several different phenomena. These include:
Coalescence:
an irreversible increase in the size of fat globules and a loss of identity of the coalescing globules;
Flocculation:
a reversible (with minor energy input) agglomeration/clustering of fat globules with no loss of identity of the globules in the floc; the fat globules that flocculate ; they can be easily redispersed if they are held together by weak forces, or they might be harder to redisperse to they share part of their interfacial layers;
Partial coalescence:
an irreversible agglomeration/clustering of fat globules, held together by a combination of fat crystals and liquid fat, and a retention of identity of individual globules as long as the crystal structure is maintained (i.e., temperature dependent, once the crystals melt, the cluster coalesces). They usually come together in a shear field, as in whipping, and it is envisioned that the crystals at the surface of the droplets are responsible for causing colliding globules to stick together, while the liquid fat partially flows between they and acts as the “cement”. Partial coalescence dominates structure formation in whipped, aerated dairy emulsions, and it should be emphasized that crystals within the emulsion droplets are responsible for its occurrence.
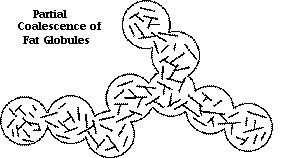
A good reference for more information on fat globules can be found in Walstra or Fox and McSweeney, Lipids.
Functional Properties
Like all fats, milkfat provides lubrication. They impart a creamy mouth feel as opposed to a dry texture. Butter flavour is unique and is derived from low levels of short chain fatty acids. If too many short chain fatty acids are hydrolyzed (separated) from the triglycerides, however, the product will taste rancid. Butter fat also acts as a reservoir for other flavours, especially in aged cheese. Fat globules produce a ‘shortening’ effect in cheese by keeping the protein matrix extended to give a soft texture. Fat substitutes are designed to mimic the globular property of milk fat. The spreadable range of butter fat is 16-24° C. Unfortunately butter is not spreadable at refrigeration temperatures. Milk fat provides energy (1g = 9 cal.), and nutrients (essential fatty acids, fat soluble vitamins).

