Glossary of Terms
Boiling Point Elevation: One of the colligative properties. The boiling point of a solution is increased over that of water by the presence of solutes, and the extent of the increase is a function of both concentration and molecular weight.
Colligative Properties: Properties which depend on the number of molecules in solution, a function of concentration and molecular weight, rather than just on the total percent concentration. Such properties include boiling point elevation, freezing point depression, and osmotic concentration.
Emulsion: liquid droplets dispersed in another immiscible liquid. The dispersed phase droplet size ranges from 0.1 – 10 µm. Important oil-in-water food emulsions, ones in which oil or fat is the dispersed phase and water is the continuous phase, include milk, cream, ice cream, salad dressings, cake batters, flavour emulsions, meat emulsions, and cream liquers. Examples of food water-in-oil emulsions are butter or margarine. Emulsions are inherently unstable because free energy is associated with the interface between the two phases. As the interfacial area increases, either through a decrease in particle size or the addition of more dispersed phase material, i.e. higher fat, more energy is needed to keep the emulsion from coalescing. Some molecules act as surface active agents (called surfactants or emulsifiers) and can reduce this energy needed to keep these phases apart.
Foam: a gas dispersed in a liquid where the gas bubbles are the discrete phase. There are many food foams including whipped creams, ice cream, carbonated soft drinks, mousses, meringues, and the head of a beer. A foam is likewise unstable and needs a stabilizing agent to form the gas bubble membrane.
Freezing point depression: of a solution is a colligative property associated with the number of dissolved molecules. The lower the molecular weight, the greater the ability of a molecule to depress the freezing point for any given concentration. For example, in ice cream manufacturing, monosaccharides such as fructose or glucose produce a much softer ice cream than disaccharides such as sucrose, if the concentration of both is the same.
Osmotic pressure: A chemical force caused by a concentration gradient. It is a colligative property and the principle behind membrane processing.
pH: is a measure of the activity of the hydronium ion (H3O+) which, according to the Debye-Huckel expression, is a function of the concentration of the hydronium ion [H3O+], the effective diameter of the hydrated ion and the ionic strength (µ m) of the solvent. For solutions of low ionic strength (µ m < 0.1) hydronium ion activity is nearly equivalent to [H3O+] which is normally abbreviated to [H+]. Then, for a weak acid (HA) dissociating to H+ and A- with a dissociation constant, Ka and pKa equal to -log10 Ka, the most important relationships are defined the following two equations:
Ka = [H+] [A-] / [HA]
pH = log 1 / [H+] = pKa + log [A-] / [HA]
Reynold’s Number: a dimensionless expression used in predicting flow patterns:
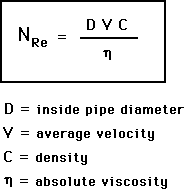
Stoke’s Equation:The velocity at which a sphere will rise or fall in a liquid varies as the square of its diameter:
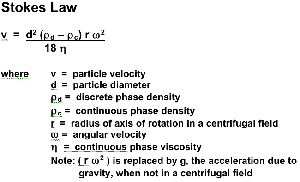
For example, a fat globule with a diameter of 2 microns will rise 4 times faster than a fat globule with a diameter of 1 micron.
Titratable acidity: A measure of titratable hydrogen ions. Includes H+ ions free in solution and those associated with acids and proteins.

