Ice Cream Structure
31 Ice Cream Shelf-Life
The most frequently occurring textural defect in ice cream is the development of a coarse, icy texture. Iciness is also the primary limitation to the shelf life of ice cream and probably also accounts for countless lost sales through customer dissatisfaction with quality. There is no answer to the question “What is the shelf-life of ice cream?”, it depends entirely on its conditions of storage. It might be one year, or it might be two weeks or less. Although the source of and the contributing factors to the problem of icincess are well known, it is also one of the defects about which I am most often asked.
Processor’s have known for a long time how to prevent iciness and the answer is still the same: formulate the ice cream properly to begin with, freeze the ice cream quickly in a well-maintained barrel freezer, harden the ice cream rapidly, and avoid as much as possible temperature fluctuations during storage and distribution. Ice crystals need to be numerous and of small, uniform size so they are not detected when eaten. It is heat shock, large temperature fluctuations, which is the greatest culprit to the loss of these small, uniform ice crystal size distributions and resulting coarse, icy texture. Perhaps it is time another message was added to the prevention of iciness and that is to educate the retailer’s and the consumer about the causes of iciness and preventative action to maintain a smooth-textured ice cream.
Before we begin looking specifically at shelf-life, you need to re-acquaint yourself with the freezing aspects of ice cream manufacturing, the structure of ice crystals in ice cream, and the theoretical aspects of the freezing process.
Temperature Fluctuations and Ice Recrystallization
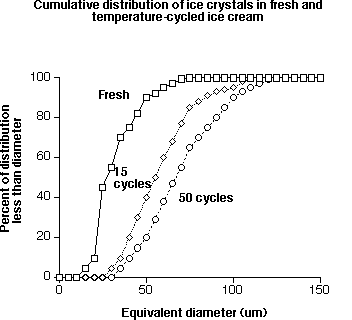
Ice crystals are relatively unstable, and during frozen storage, they undergo changes in number, size, and shape, known collectively as recrystallization. This is probably the most important reaction leading to quality losses in all frozen foods. Some recrystallization occurs naturally at constant temperatures, but by far the majority of problems are created as a result of temperature fluctuations. If the temperature during the frozen storage of ice cream increases, some of the ice crystals, particularly the smaller ones, melt and consequently the amount of unfrozen water in the serum phase increases. Conversely, as temperatures decrease, water will refreeze but does not renucleate. Rather, it is deposited on the surface of larger crystals, so the net result is that the total number of crystals diminish and the mean crystal size increases. Temperature fluctuations are common in frozen storage as a result of the cyclic nature of refrigeration systems and the need for automatic defrost. However, mishandling of product is probably the biggest culprit. The sight of ice cream sitting unrefrigerated on a loading dock, in the supermarket aisle, in a shopping cart, or in someone’s grocery bag is too common. If one were to track the temperature history of ice cream during distribution, retailing, and finally consumption, one would find a great number of temperature fluctuations. Each time the temperature changes, the ice to serum content changes, and the smaller ice crystals disappear while the larger ones grow even larger. Recrystallization is minimized by maintaining low and constant storage temperatures.
The graph below provides data to show the increase in size of ice crystals that occurs with temperature cycles (from the work of A. Flores and H. D. Goff).
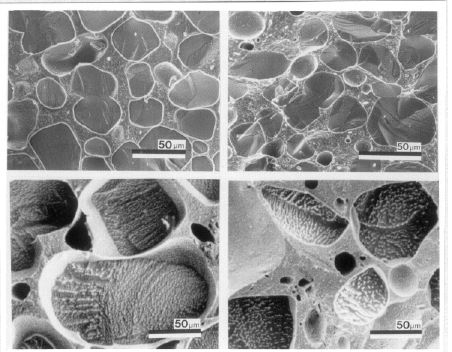
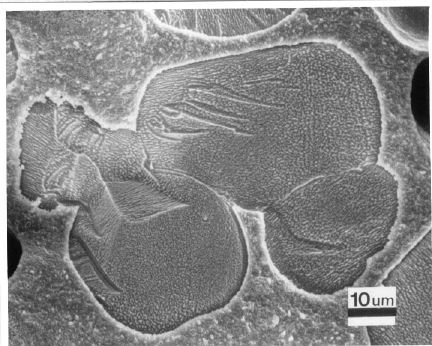
Below are several cryo-scanning electron micrographic images of ice cream after temperature fluctuations. In the first composite, all pictures are at the same magnification, the top two are fresh, the bottom two are heat-shocked. You can see the tremendous increase in crystals size that has occurred. The next image shows an example of accretion, where crystals fuse as they grow.
The Role of Stabilizers
The ice cream stabilizers, locust bean gum, guar gum, carboxymethyl cellulose, sodium alginate, carrageenan, and xanthan, are a group of ingredients used commonly in ice cream formulations. They are usually integrated with the emulsifiers in proprietary blends. The primary purposes of using stabilizers in ice cream are to produce smoothness in body and texture, retard or reduce ice and lactose crystal growth during storage, and to provide uniformity of product and resistance to melting. Additionally, they stabilize the mix to prevent wheying off, produce a stable foam with easy cut-off at the barrel freezer and slow down moisture migration from the product to the package or the air. The action of the polysaccharides in ice cream result from their ability to form gel-like structures in water and to hold free water. Control of iciness by stabilizers has been attributed to a reduction in the growth of ice crystals over time, probably related to a reduction in water mobility as water is entrapped by their entangled network structures in the serum phase. Proper formulation with stabilizers designed to combat against heat shock is an almost essential defense against the inevitable growth of ice crystals. Low total solids mixes are also more difficult to effectively stabilize as the increased content of water leads to more ice at any given temperature. Also, high concentrations of sugars or lactose will change the ratio of water to ice and lead to greater problems of recrystallization.
Education needed
I hope I have stimulated ice cream processors to begin an education campaign for ice cream retailers and consumers about the subject of heat shock and coarseness. I often hear processors say that handling of the product after it leaves their hands is out of their control. Do not forget, however, that the consumer is buying your label of product. The quality they receive is a reflection on you, despite where the damage occurred. The people unloading or stocking your ice cream, and the customer who buys your ice cream cannot be expected to understand the concepts of ice crystal size distributions and ice crystal growth without a little help from you. Ice cream is unlike the other frozen foods they handle routinely and this must be explained to them. We often sell ice cream at the University of Guelph. The majority of our customer’s comment on the superb texture of our ice cream. They often ask us what we do differently from other manufacturer’s to produce an ice cream that is so smooth. Although we would like to take credit for some great revelation in processing, the difference is that they are buying ice cream that is fresh, directly from our hardening room. If customer’s were buying ice cream from the hardening room’s of all manufacturer’s, no doubt you would get the same comments, but they are not.
That is why I am suggesting some education to retailers and consumers on the subject may be of benefit to both them and you. An information package to retailers on proper handling of ice cream may be very welcome so that they can use it in their training of new and continuing employees. The IICA in Washington has prepared material for this purpose. Consumers can be contacted through side panels on ice cream packages or through point of purchase displays. Whatever the media, the message is that both retailers and consumers can play an important role in maintaining the texture in ice cream which is desired.
Maintaining Shelf-life
- Formulate the ice cream properly
- Freezing point depression and sugar considerations
- Stabilizers
- Freeze the ice cream quickly in a well-maintained barrel freezer
- Continuous freezers with high rates of heat exchange
- Free of fouling (eg., oil) on refrigerant side
- Blades with a good, even edge
- Short, insulated process lines through ingredient feeder, packaging equipment
- Precooling of ingredients
- Harden the ice cream rapidly
- High rates of heat transfer: convection (high ΔT and forced air with free air flow) or conduction
- Importance of thermal centre and shrink-wrapping of bundles
- Avoid temperature fluctuations during storage and distribution
- Importance of low, constant temperatures
- Avoid mishandling at all stages
- Educate retailers and consumers about shelf stability
- Mishandling is usually not at the manufacturing level but quality losses affect consumer acceptance of your product.

