Ice Cream Mix Ingredients
13 Stabilizers
The stabilizers are a group of compounds, usually polysaccharide food gums, that are responsible for adding viscosity to the mix and the unfrozen phase of the ice cream. This results in many functional benefits, listed below, and also extends the shelf life by limiting ice recrystallization during storage. Without the stabilizers, the ice cream would become coarse and icy very quickly due to the migration of free water and the growth of existing ice crystals.
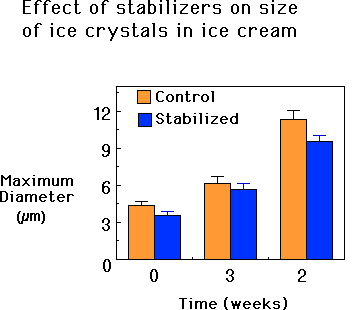
The smaller the ice crystals in the ice cream, the less detectable they are to the tongue. Especially in the distribution channels of today’s marketplace, the supermarkets, the trunks of cars, and so on, ice cream has many opportunities to warm up, partially melt some of the ice, and then refreeze as the temperature is once again lowered (see also the discussion on the fundamental aspects of freezing and ice cream shelf life for a more in-depth look at this process, and some discussion regarding the role of stabilizers in inhibiting it). This process is known as heat shock and every time it happens, the ice cream becomes more icy tasting. Stabilizers help to prevent this.
The functions of stabilizers in ice cream are:
- In the mix: To stabilize the emulsion to prevent creaming of fat and, in the case of carrageenan, to prevent serum separation due to incompatibility of the other polysaccharides with milk proteins, also to aid in suspension of liquid flavours
- In the ice cream at draw from the scraped surface freezer: To stabilize the air bubbles and to hold the flavourings, e.g., ripple sauces, in dispersion
- In the ice cream during storage: To prevent lactose crystal growth and retard or reduce ice crystal growth during storage (see also the discussion on ice cream shelf life, which discusses the mode of action of stabilizers in affecting ice recrystallization), also to prevent shrinkage from collapse of the air bubbles and to prevent moisture migration into the package (in the case of paperboard) and sublimation from the surface
- In the ice cream at the time of consumption: To provide some body and mouthfeel without being gummy, and to promote good flavour release
- (Note: all of the above, except perhaps for their role in retarding ice crystallization, can be attributable to the viscosity increase in the unfrozen phase of the ice cream)
Limitations on their use include:
- production of undesirable melting characteristics, due to too high viscosity
- excessive mix viscosity prior to freezing
- contribution to a heavy or chewy body
The stabilizers in use today include:
Locust Bean Gum:
soluble fibre of plant material derived from the endosperm of beans of exotic trees grown mostly in Africa (Note: locust bean gum is a synonym for carob bean gum, the beans of which were used centuries ago for weighing precious metals, a system still in use today, the word carob and Karat having similar derivation)
Guar Gum:
from the endosperm of the bean of the guar bush, a member of the legume family grown in India for centuries and now grown to a limited extent in Texas
Carboxymethyl cellulose (CMC):
derived from the bulky components, or pulp cellulose, of plant material, and chemically derivatized to make it water soluble
Xanthan gum:
produced in culture broth media by the microorganism Xanthaomonas campestris as an exopolysaccharide, used to a lesser extent
Sodium alginate:
an extract of seaweed, brown kelp, also used to a lesser extent
Carrageenan:
an extract of Irish Moss or other red algae, originally harvested from the coast of Ireland, near the village of Carragheen but now most frequently obtained from Chile and the Phillipines
Each of the stabilizers has its own characteristics and often, two or more of these stabilizers are used in combination to lend synergistic properties to each other and improve their overall effectiveness. Guar, for example, is more soluble than locust bean gum at cold temperatures, thus it finds more application in HTST pasteurization systems. Carrageenan is not used by itself but rather is used as a secondary colloid to prevent the wheying off of mix which is usually promoted by one of the other stabilizers.
Gelatin, a protein of animal origin, was used almost exclusively in the ice cream industry as a stabilizer but has gradually been replaced with polysaccharides of plant origin due to their increased effectiveness and reduced cost.

