Ice Cream Structure
27 Colloidal aspects of ice cream structure
Please look at the following two diagrams when reading the description of structure formation below, and try to put the two together in your mind.
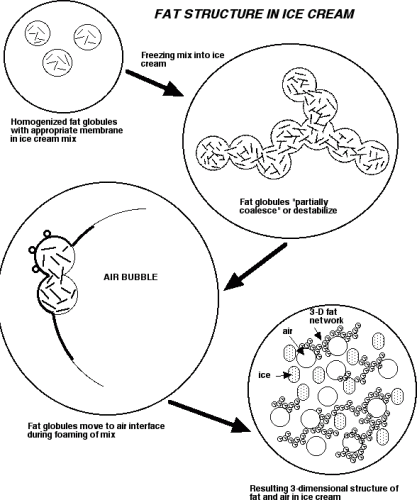
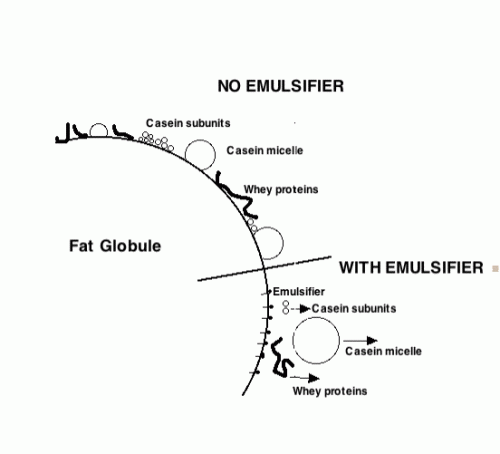
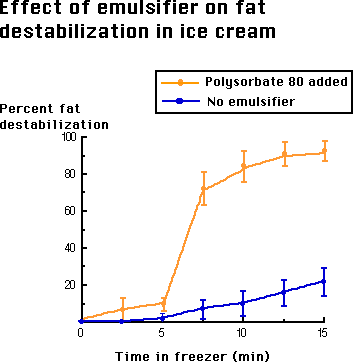
Ice cream is both an emulsion and a foam. The milkfat exists in tiny globules that have been formed by the homogenizer. There are many proteins that act as emulsifiers and give the fat emulsion its needed stability. The emulsifiers are added to ice cream to actually reduce the stability of this fat emulsion by replacing proteins on the fat surface (shown on the right), leading to a thinner membrane more prone to coalescence during whipping. When the mix is subjected to the whipping action of the barrel freezer, the fat emulsion begins to partially break down and the fat globules begin to flocculate or destabilize. The air bubbles which are being beaten into the mix are stabilized by this partially coalesced fat. If emulsifiers were not added, the fat globules would have so much ability to resist this coalescing, due to the proteins being adsorbed to the fat globule, that the air bubbles would not be properly stabilized and the ice cream would not have the same smooth texture (due to this fat structure) that it has.
This fat structure which exists in ice cream is the same type of structure which exists in whipped cream. When you whip a bowl of heavy cream, it soon starts to become stiff and dry appearing and takes on a smooth texture. This results from the formation of this partially coalesced fat structure stabilizing the air bubbles. If it is whipped too far, the fat will begin to churn and butter particles will form. The same thing will happen in ice cream which has been whipped too much.

