Gas Exchange and Transport
This gas exchange section seems to be one of the most commonly identified sticking points within human gas physiology. Here is where we dive into how gas exchange occurs at the lung as well as the level of skeletal muscle. We also investigate what drives this process. This section aims to look at the lungs within different magnifications to determine how the body reacts to gas exchange and transport at various levels. Within this section, multiple learning objects will work to enhance subject comprehension rather than memorization which will be aided if you practice what would happen in various scenarios.
Learning Outcomes
In this section you will learn…
- How to describe the lung and tissue environment and how this impacts oxygen and hemoglobin binding.
- Why PO2 and PCO2 within the alveoli do not reflect that of the environment.
- How oxygen and carbon dioxide are stored in the blood.
- How the PvCO2 might differ within someone who was working out versus studying, and why this is.
- What the equations associated with red blood cells and blood plasma are.
How are Oxygen and Carbon Dioxide Exchanged at the Lung?
Oxygen will travel along its concentration gradient from the alveoli, which have a high alveolar partial pressure (PAO2), to the arteries with a lower arterial partial pressure (PaO2). A small percentage of the oxygen can be transported in the blood dissolved in plasma. But the majority of oxygen is transported bound to hemoglobin. It is important to note that only oxygen dissolved in the plasma is ready for exchange at the tissue level, so hemoglobin reversibly binds with the oxygen to be dropped off at the tissue level. But we will talk about the tissues in a little while, so put this thought on hold for now!
Carbon dioxide will also travel along its concentration gradient from the high venous partial pressure (PvCO2) to low alveolar partial pressure (PACO2), allowing us to exhale excess carbon dioxide. Multiple different transportation platforms carry carbon dioxide. It can be transported in plasma bound to hemoglobin through a process called carbaminohemoglobin and it can also be stored in bicarbonate or bound to plasma proteins. To allow carbon dioxide to be exchanged at the lung level, it must be released from the plasma proteins and bicarbonate.
Remember that the lung’s environment encourages the binding of oxygen (O2) to hemoglobin due to the high pressure of oxygen (PO2). The low carbon dioxide (CO2) in the lung is also a driving factor for unloading the CO2 that has been stored in the blood.
Test Your Knowledge
Real-Life Scenario:
It’s a beautiful snowy day outside. Fluffy flakes of snow slowly fall as you look out your bedroom window. You decide to take this as a sign to enjoy some hot chocolate and snuggle up to watch a seasonal movie. After the snow has stopped, you choose to go outside for a run to get out of your groggy hot chocolate coma. What would happen to your capillary and venous oxygen levels? Would this increase or decrease the drive for diffusion at the level of the tissue? Hint: You went from a stationary position to a stimulated movement very quickly, so how will that fast change affect you?
Processes and Equations in the Lung
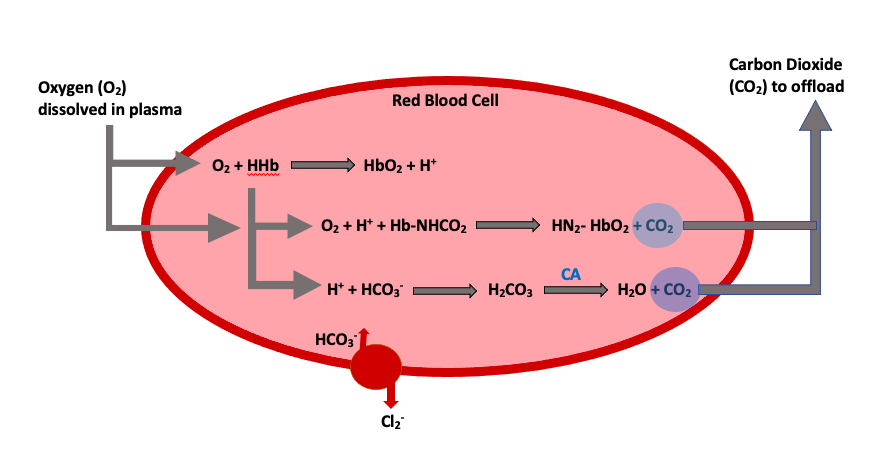
Knowing and understanding the processes that occur to allow for oxygen transport throughout the body is crucial. Let’s take a look at the various locations involved with the oxygen exchange and how it occurs. There are four equations that you need to know for this unit, this is usually what scares students, but they are easy if you think through them (Figure 1).
We will start by looking at what happens in red blood cells. This first equation showcases oxygen storage within a hemoglobin molecule (HbO2) on the product’s side of the equation. This is produced by the free oxygen bumping off the first hydrogen ion (H+) within the hemoglobin compound. During this exchange process, the newly available hydrogen ion is left as a product substrate from the oxyhemoglobin reaction.
[latex]O_2 + HHb → HbO_2 + H^+[/latex]
The following equation showcases the process of carbon dioxide release from the bound transport red blood cells (Hb·NHCO2) into the plasma. We see oxygen being bound to hemoglobin on the product’s side of the reaction (NH2·HbO2). In this scenario, oxygen acts to replace the bound carbon dioxide, to release it into the plasma. You might also notice that the extra hydrogen ion from the equation above now plays a role in binding oxygen.
[latex]O_2 + H^+ + Hb·NHCO_2 → NH_{2}·HbO_{2} + CO_2[/latex]
In our final red blood cell equation, we see an alternative transportation method for carbon dioxide combined with bicarbonate (HCO3−). Again, we can see the role that the free hydrogen ion plays in binding to bicarbonate (H+ + HCO3−) to form carbonic acid (H2CO3) and eventually be released into the plasma as carbon dioxide and water. This released form of carbon dioxide and water (H2O) allows for exchange at the level of the lung. It is important to note that two key players catalyze and transport carbon dioxide. The first is an enzyme known as carbonic anhydrase (CA), which is present in the red blood cell and allows for quick stripping of carbon dioxide from the carbonic acid molecule. The second is a chloride shift (transporter), which brings bicarbonate into the cell to ensure that there is enough substrate to allow the release of carbon dioxide.
[latex]H^+ + HCO_{3}^− → H_{2}CO_{3} → H_{2}O + CO_2[/latex]
Test Your Knowledge
Clinical Application:
Describe what happens in the blood to allow oxygen and carbon dioxide to be exchanged when systemic blood supplies the tissues of the body. Hint: Make sure to include all the reactions that take place in the red blood cell and the plasma.
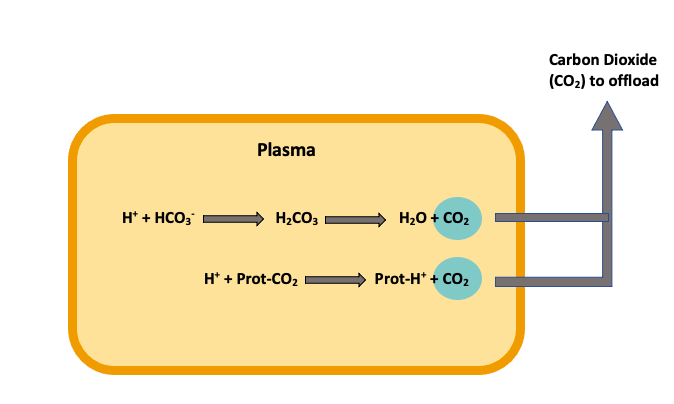
Now that we understand what occurs within the red blood cells let’s look at what exchanges occur within the plasma. This first equation might look familiar. That’s because we already saw this equation within the red blood cell. The only difference between these two equations is that there is no carbonic anhydrase (CA) within the plasma, so this reaction is much slower.
[latex]H^+ + HCO_{3}^− → H_{2}CO_{3} → H_{2}O + CO_2[/latex]
The final way carbon dioxide can be bound and transported is with a protein’s help (Prot·CO2). When the free hydrogen ion interacts with the protein-carbon dioxide complex, a displacement occurs, releasing the carbon dioxide and forming a hydrogen-protein complex (Prot·H+). This process releases carbon dioxide into the plasma, allowing for exchange to happen at the lungs’ level (Figure 2).
[latex]H^+ + Prot·CO_2 → Prot·H^+ + CO_2[/latex]
Test Your Knowledge
Real-Life Scenario:
Two friends decide to have a breath-holding contest. The one friend sits silently waiting for the contest to begin and then takes one big breath in before starting. The other friend hyperventilates up until the start of the contest. Assuming they have approximately the same health status, who do you expect will win? Hint: How would an increase in air consumption alter the friends’ physiology.
What is Happening at the Level of the Tissue?
The tissue environment is the opposite of that at the lung due to tissue metabolism, creating a low PO2 and high PCO2 micro-climate. In this scenario, oxygen will travel down its concentration gradient from an area of high concentration within the systemic arteries to a place of low concentration within the tissue mitochondria. In turn, carbon dioxide will move down its concentration gradient from a high concentration area within the tissues to a low concentration within the systemic veins.
The equations learned earlier in this unit will all apply at the tissue level as well. However, the direction of the equations must be flipped to demonstrate the storage of carbon dioxide and the release of oxygen.
Tips From Past Students
It might be helpful to apply this situation to a real-life example. If someone is active, then we would expect an increase in tissue metabolism. Resulting in increased use of oxygen and production of carbon dioxide waste. Overall, this would increase the drive for gas exchange at the tissues.
The Oxygen Dissociation Curve
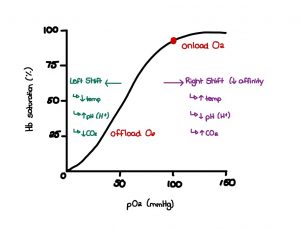
The oxygen dissociation curve is a graph that depicts oxygen partial pressure along the horizontal x-axis and saturation on the vertical y-axis, as seen in figure 3. Carbon dioxide and oxygen are transported within the blood following fluctuations in partial blood pressure. Most oxygen is carried in the blood via hemoglobin found within red blood cells, as we discussed earlier. To review, hemoglobin strongly binds up to four oxygen molecules to allow for easy transport from the lungs to the tissues. However, when the oxygenated red blood cells arrive at the desired tissue, the bound oxygen has to become available and dissolved into the blood plasma. To make this happen, there has to be a change within the tissue environment.
For instance, at the tissue level, we want to promote the dissociation of oxygen from hemoglobin. The leading factor contributing to this dissociation is the decrease in PO2 to 40 mmHg, as seen in the graph below. This decrease in pressure from 100mmHg at the lungs creates a partial pressure gradient at the tissue level that increases the rate of oxygen diffusion from high pressure within the red blood cells to low pressure within the tissue. The available receptors are then readily accessible for carrying other metabolic waste material back to the lungs for proper disposal. There are additional factors that contribute to this dissociation environment as well. They are outlined below.
An increase in aerobic metabolism contributes to a rise in carbon dioxide concentration at the tissue level. This high concentration of CO2 will automatically travel to an area of lower concentrations if given the opportunity, which is presented when oxygen-saturated red blood cells arrive. This pressure gradient encourages oxygen dissociation and CO2 replacement. The increase in hydrogen ions produced from contracting skeletal muscles makes the tissue an acidic environment that contributes to dissociation. In addition, an increase in temperature increases dissociation. Finally, an increase in anaerobic by-products such as 2, 3 diphosphoglycerate (2, 3 DPG) enhances oxygen association.
Test Your Knowledge
Real-Life Scenario:
You just got on a roller coaster for the first time in your life. You are super excited about the massive drop that comes at the end. At the same time, you can feel your heart pounding in your chest. Having a deep understanding of blood gas physiology, you understand that you are experiencing increased blood flow across your pulmonary capillary bed. Would your alveolar partial pressures of CO2 and O2 increase or decrease due to this increase in pulmonary blood flow? Hint: Think about how your ventilation and blood flow are being impacted. Utilize the dissociation curve for visualization help.
This tissue environment works together to decrease hemoglobin’s ability to bind any remaining oxygen. An inverse environment can be seen at the level of the lungs. Try the activity below to see if you can determine what this environment might look like.
The following video is included as a review for some concepts already covered in the course and is an excellent resource for better understanding the oxygen dissociation curve. The first 2:30 spend time reviewing respiratory anatomy and circulation. Next, from 2:31-4:55 is above course level as it describes how oxygen is carried bound to hemoglobin. At 4:56 partial pressures of oxygen and carbon dioxide are described. Then at 5:58-8:32 the video talks about the oxygen dissociation curve. Lastly, from 8:33-11:52 the factors leading to dissociation are taught. Key points you should take away from this video include that hemoglobin can carry 4 oxygen molecules and in the lung, hemoglobin has a high affinity for oxygen. You should also be able to describe how the lung and tissue environments influence oxygen affinity.
Are you interested in learning about how a mammal’s body size can affect the properties of its dissociation curve? For example, it has been shown that smaller mammals are more easily able to release oxygen to the tissue. Meaning a small drop in PO2 under 100 mmHg leads to a large drop in hemoglobin saturation. It has also been shown that those living at altitude will have a left shift in their oxygen dissociation curve. This means that the hemoglobin holds onto the oxygen more tightly. This has been seen in llamas! [1]
Tips From Past Students
Understanding how the alterations between the lungs and tissue’s micro-environments will help you understand how physiological states are impacted. For instance, how a state of decreased blood flow due to a medical condition like congestive heart failure impacts oxygen and carbon dioxide partial pressures. Also, learning the equations and understanding the different diagrams can be tricky. Try drawing from memory, writing key summary points for what happens at the lung and tissue, explaining the diagram to a friend, and having someone else define the diagram to you! The more ways you can review the material, the better.
Approaching the Processes for Gas Exchange and Transport Differently
The following video is included as a review of gas exchange and transport. This video logically goes step by step through the processes of gas transport and exchange. It is great for those who are visual and auditory learners, as there are lots of drawings. This learning object is especially helpful in understanding the origin of the equations within this chapter. The video starts by reviewing the respiratory and circulatory system’s anatomy. At 3:18-8:40 gas exchange between the blood and tissue is discussed. From 8:41-13:30 it reviews the gas exchange that takes place at the level of the lung. Please note that the direction of the equations are opposite of those outlined in the notes for the lung, seen earlier in this subchapter. As an extra piece of information, this video also discusses the importance of carbonic anhydrase and the chloride shift. Key takeaways from the video are how oxygen and carbon dioxide are transported in the blood, how oxygen enters the tissue, and how carbon dioxide enters the alveoli and is released into the blood to be transported.
Key Takeaways
Consider the following concepts to help guide your studies:
- There is higher PO2 pressure at the lung level, allowing for O2 to reversibly saturate all hemoglobin molecules that pass by in red blood cells.
- Carbon dioxide has a variety of transportation methods that encourage gas exchange and waste removal.
- There are multiple reactions within red blood cells and the plasma that allow for gas exchange to occur.
- The micro-environments in the lungs and tissues are very different regarding hydrogen ion concentration, temperature, and carbon dioxide pressure.
- Blood flow, oxygen inhalation, and carbon dioxide exhalation rates significantly impact the pressure gradients that stimulate gas exchange.
Subchapter Quiz
The questions below have been created to assess your knowledge within this subchapter. You will find multiple-choice questions as well as open-ended questions to test your understanding. Attempt these without your notes to narrow down any concepts you may need to revisit in your studying.
Media Attributions
- Gas Exchange at the Lung © Allanna MacKenzie is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Blood Plasma CO2 Exchange © Allanna MacKenzie is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Oxygen Dissociation Curve © Leah Martin is licensed under a CC BY-ND (Attribution NoDerivatives) license
- Schmidt-Neilson, K., & Larimer, J. L. (1958). Oxygen dissociation curves of mammalian blood in relation to body size. American Journal of Physiology, 195(2), 424-428. ↵
A protein responsible for transporting oxygen in the blood. It's molecule is comprised of four subunits, each containing an iron atom bound to a heme group.
A compound of hemoglobin and carbon dioxide, one of the forms in which carbon dioxide exists in the blood.
An enzyme present in the RBC that catalyzes the stripping of carbon dioxide from bicarbonate.
Brings bicarbonate into the cell to ensure that there is enough substrate to allow release of carbon dioxide.

